Medical Doctor (MD) Program
Janus Kinase 1 Inhibition for the Treatment of Granuloma Annulare

Medical Doctor (MD) Program
Janus Kinase 1 Inhibition for the Treatment of Granuloma Annulare
In this open-label clinical trial, 10 patients with GA were enrolled to assess activity of the JAK1 specific inhibitor abrocitinib.
Overview
Granuloma annulare (GA) is an inflammatory skin disease without FDA-approved therapies. Prior studies have suggested that JAK inhibition may be therapeutically effective in GA, with JAK1-dependent cytokines appearing to show particular importance in disease pathogenesis. In this open-label clinical trial, 10 patients with GA were enrolled to assess activity of the JAK1 specific inhibitor abrocitinib.
Reseacher

Okeroghene Rufin, Class of 2027
MD Program
Frank H. Netter MD School of Medicine
Janus Kinase 1 (JAK1) Inhibition for the Treatment of Granuloma Annulare
Fellow researchers: Muhammad H. Junejo of Yale School of Medicine; Erica Hwang of Yale School of Medicine; Bridget Shields of University of Wisconsin; Misha Rosenbach of University of Pennsylvania; Yiwei Wang of Yale School of Medicine; Brett King of Yale School of Medicine; and William Damsky of Yale School of Medicine
Background
- Granuloma annulare (GA) is an idiopathic inflammatory disorder of the skin.
- GA affects at least 300,000 people in the United States and can impact patients’ quality of life (QoL).
- GA is most commonly characterized by pink to dull pink annular papules and plaques.
- Histologically, GA can present as palisaded granulomatous inflammation with collagen degeneration and increased mucin or as interstitial inflammation without significant extracellular matrix alteration.
In previous studies, tofacitinib, a JAK 1/2/3 inhibitor, has been shown to be effective in the treatment of GA. However, it is unclear if JAK1 specific inhibition would be effective in the treatment of GA.
Objective
Evaluate the efficacy of JAK1 specific inhibition in the treatment of granuloma annulare.
Methods
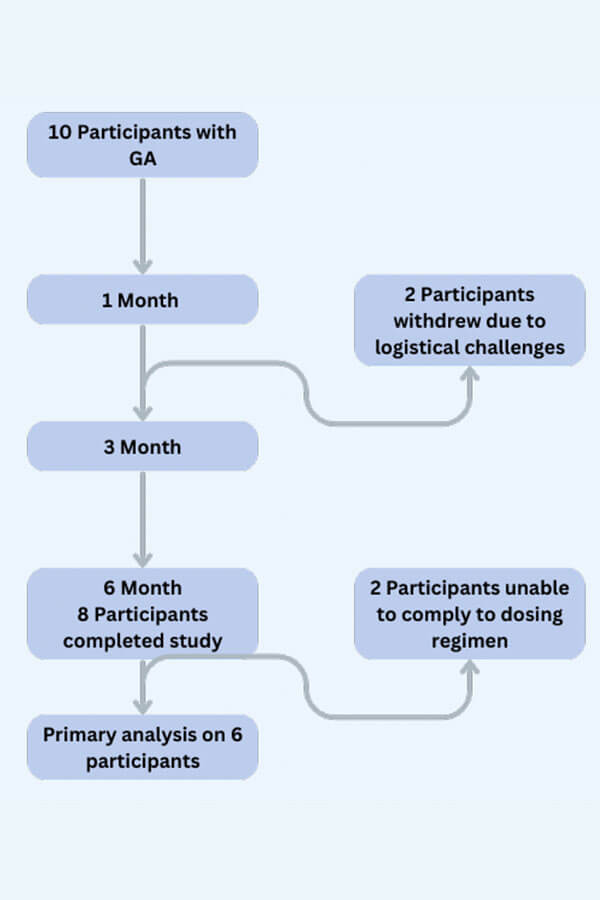
- We performed an open-label, proof-of-concept clinical trial with a Jak1 inhibitor, abrocitinib.
- Ten patients with GA were enrolled and treated with abrocitinb 200 mg daily for 6 months.
- The primary outcome was change in body surface area (BSA) involvement by clinically active GA after 6 months of abrocitinib treatment.
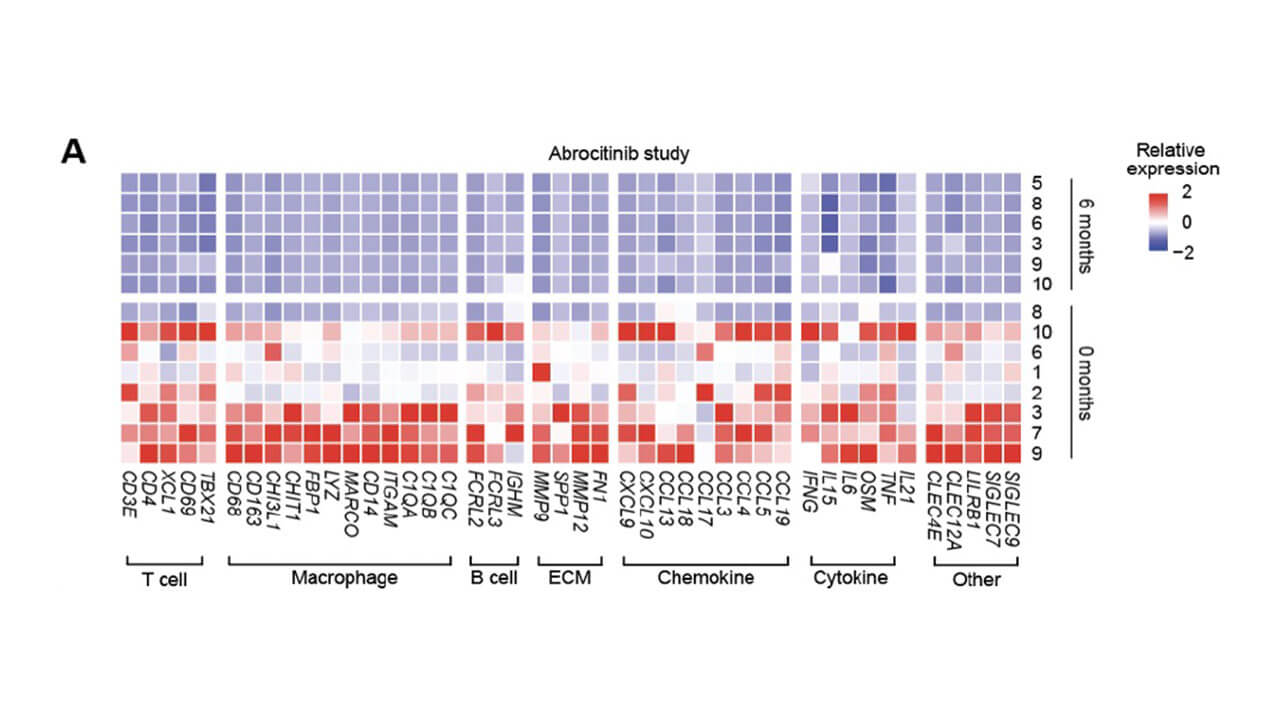
- Molecular analysis was performed using RNA-seq on lesional skin biopsies at baseline and after 6 months of treatment.
Results
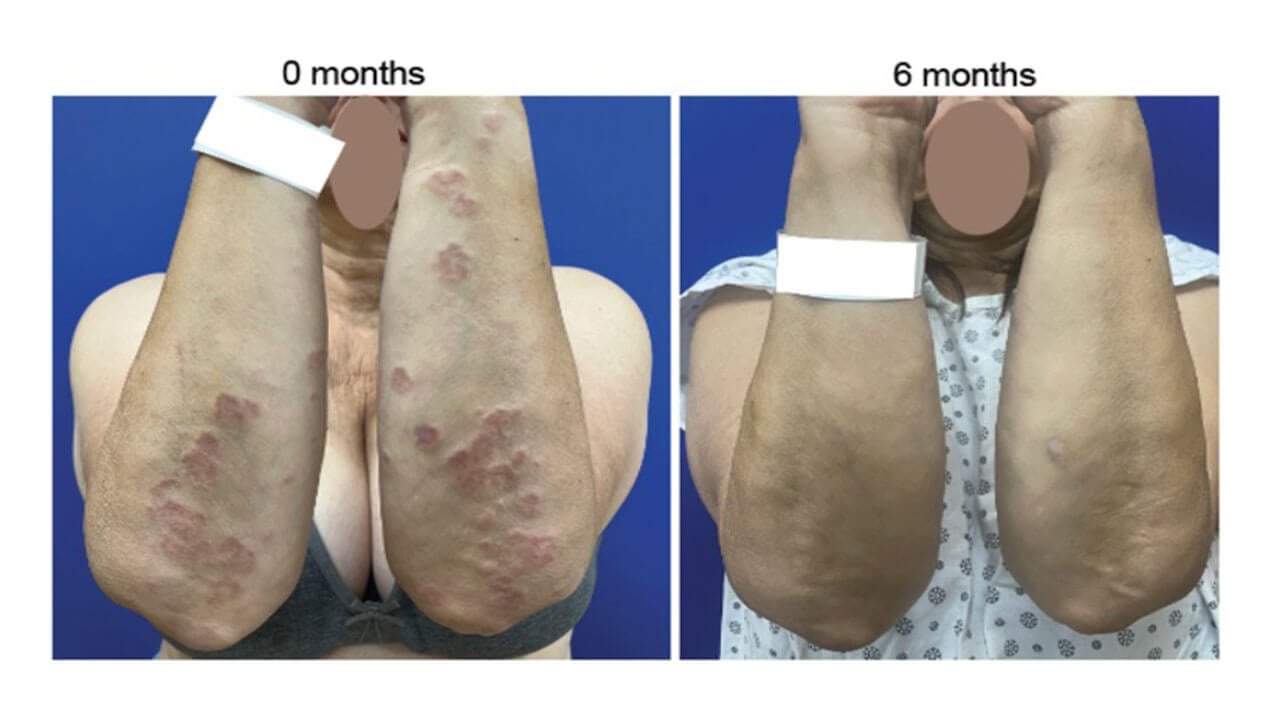
A significant reduction in average BSA involvement from 9.6% to 2.2% was observed in the 6 participants adherent to intended daily dosing.
Changes in the transcriptional profile of skin as a function of treatment were also assessed and showed potent inhibition of key JAK1-dependent cytokine activity (IFN-Ɣ, IL-15, IL-21, and oncostatin M) and other biomarkers by abrocitinib.
Conclusion
These findings suggest that JAK1 specific inhibition appears sufficient to treat GA. Larger, controlled studies are warranted in order to better understand the efficacy and safety of abrocitinib in GA.
For Further Discussion
This serves as an overview of the project and does not include the complete work. To further discuss this project, please email Okeroghene Rufin.
References
1. Piette EW, Rosenbach M. Granuloma annulare: Clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75(3):457-65.
2. Dicken CH, Carrington SG, Winkelmann RK. Generalized granuloma annulare. Arch Dermatol. 1969;99(5):556-63.
3. Piette EW, Rosenbach M. Granuloma annulare: Pathogenesis, disease associations and triggers, and therapeutic options. J Am Acad Dermatol. 2016;75(3):467-79.
4. Hwang E, Lee T, Okifo K, Murphy M, Damsky W. Retrospective assessment of immunologic and histologic heterogeneity in granuloma annulare by cytokine staining. Int J Dermatol. 2024;63(5):655-9.
